It’s fair to ask if there is any evidence for this new idea. Luckily, one of the great mysteries of modern physics has been pointing to this model all along, just no one realized it until now.
This mystery is: why is the nucleus of Hydrogen larger than a proton when the only thing in the nucleus is a proton?
The size of the nucleus of Hydrogen is determined by shooting electrons at the Hydrogen atom and seeing how they are deflected. Here is the data:
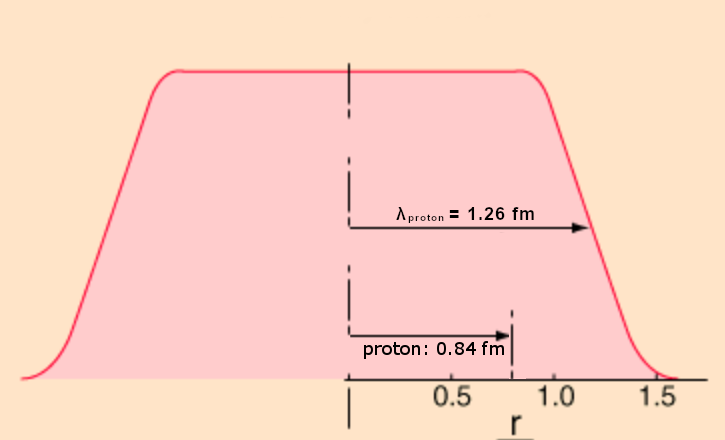
That difference between the size of the proton and the “edge” of the “nucleus” (red line) is the space that is compressed when it is displaced by the creation of the proton. The edge averages about 1.24 fm (or femtometer. One fm is 0.000000000000001 meter, or one quadrillionth of a meter.) The proton, however has a radius of 0.84 fm. (We will explain the notation λproton in a later post.)
Previous to The New Physics model, there has been no satisfactory way to explain this discrepancy between the measured size of the Hydrogen nucleus and its only occupant. But by The New Physics model, the electrons are deflected by the denser space around the particle that was compressed when the particle was inserted. This hypothesis is further supported by the fact that the measured “edge” of the nucleus is “fuzzy” between 1.0 fm and 1.5 fm, just what we would expect if the electrons were diverted by a layer of denser space rather than a hard edge.
This is very strong evidence that putting a proton into space has an effect on space itself.
But stay with us, this gets even weirder!