A little over100 years ago, the New Zealand physicist Ernest Rutherford performed an important experiment. He shot a beam of alpha particles at a foil of gold. Now alpha particles are just the nucleus of a Helium atom: two protons and two neutrons. Turns out this is a very stable nucleus so you can shoot them at things and they won’t break apart.
Anyway, the surprising result of this experiment was that most of the alpha particles passed straight through! Most of the gold atom turned out to be empty. A few very light particles called electrons orbited a tiny gold nucleus; 99.98% of the weight of the gold atom was in its tiny nucleus, but the gold atom was 20,000 times larger than its nucleus! It seemed to look like this:
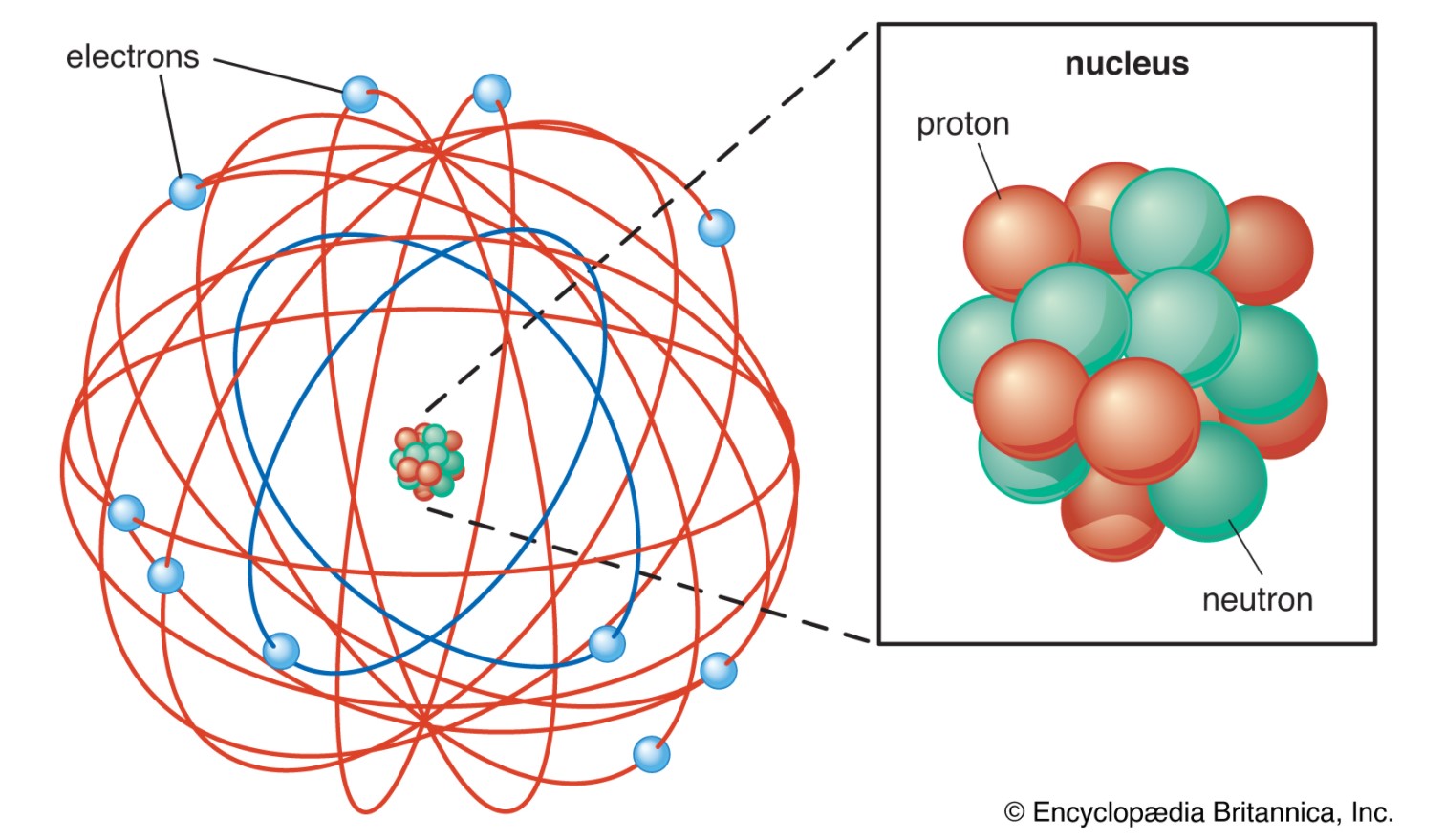
This is definitely not a scale drawing, since the atom here is way smaller than than 20,000 of the size of the nucleus, but it sort of shows how empty the gold atoms are. If we did have a drawing to scale, you couldn’t see the nucleus at all.
That the things around us (even ourselves) were far from solid was a pretty disturbing revolution in physics in its day. In fact it is still pretty amazing, not something we think about much.
If atoms are mostly empty, why do things appear to be solid? Why don’t we fall through the floor?
The reason is that the outside of atoms is made of rapidly moving electrons. Electrons have a negative electrical charge, and the electrons of one atom naturally repel the electrons of another, because negative charges repel each other. Since our eyes aren’t good enough to see the individual atoms, much less their interiors, things appear to be solid to us, and because the electrons in our bodies are repelled by the electrons in the floor, we don’t fall through.
The New Physics takes this concept of emptiness to a new level, and as such it is every bit as revolutionary as the gold foil experiment of 100 years ago. Now, despite the nucleus having 99.98% of the mass of the gold atom, even the particles in the nucleus are 99.5% empty! And it will turn out that the remaining 0.5%–the quarks–make no direct contribution to mass! We barely had time to adjust to the concept that atoms are mostly empty, and now we have to swallow the notion that the nucleus particles are also empty!
Give us a break!!!
And if the particles are all empty, how can they have mass? We’ll explain everything, we promise! It will change the way you view the world around you forever. And it will all make perfect sense. So stay with us, all will be revealed (well, as much as we know so far, anyway.)